 YuYi
YuYi
 Oct 14,2023
Oct 14,2023

Mild traumatic brain injury (mTBI) can cause abnormal production of free radicals and is associated with oxidative stress, secondary injury signaling cascades, mitochondrial dysfunction, and adverse functional outcomes. Antioxidants pharmacologically targeting free radicals have emerged as proven treatments. Bailey et al. from Virginia Tech University in the United States first reported a new type of regenerated cerium oxide nanoparticle antioxidant that can reduce neuronal death and calcium dysregulation, protect the endogenous antioxidant system, and improve cognition in vivo and in vitro neurotrauma models. Function. The results were published in the "Journal of Neurotrauma" in June 2020.
Secondary injuries in mild traumatic brain injury (mTBI) include abnormal production of free radicals and increased oxidative stress. Despite the presence of endogenous brain antioxidants such as superoxide dismutase (SOD), catalase, and glutathione (GSH), excessive free radical production after TBI exceeds endogenous defense limits and puts the brain in an An abnormal state of high oxidative stress that continues into a chronic stage. This increase in oxidative stress can cause changes in cognitive abilities. Traditional antioxidants have been used in an attempt to mitigate the pathological effects of TBI, but have had limited success in clinical trials.
Cerium is a rare earth element in the lanthanide series that has multiple valence states and mediates redox activities. The lattice structure of oxides has greater redox capabilities due to electron "holes" or oxygen defects in the nanoparticle matrix. Furthermore, the free radical scavenging activity of CeONPs is reproducible under biological conditions, allowing sustained activity. Research shows that CeONPs preserve the life cycle of brain cells and preserve normal calcium signaling. CeONPs also protect neurons and other cell types from free radical stimulation and reduce inflammatory function.
CeONPs were resuspended in citrate buffer. The distribution of nanoparticles in the brain was confirmed using rats (n = 6) using a single tail vein injection. Neuronal cultures were prepared from 1-2 day-old neonatal rats and grown in silicone-bottomed tissue culture plates, and the cells were damaged using the 94A cell damage controller model. These injuries have been shown to be related to rotational acceleration-deceleration injuries occurring in humans. On days 12-14 of in vitro culture, CeONP was added to the culture. Neuronal damage was assessed by measuring the uptake of propionic sodium iodide (PrI) and counting damaged cells. [Ca2+] was determined by microspectrophotometric imaging.
Brain injury was induced in adult male SD rats through a mild lateral hydraulic impact model (MFP). The CeONP administration pattern was as follows: 3 administrations within 3 hours after injury (3×) or 5 administrations within 48 hours after injury (5×). The 3× group received injections at 1 min, 15 min, and 3 h after injury, and the 5× group received injections at 24 h and 48 h on this basis. The low dose group is 0.05 μg/g (LD) and the high dose group is 0.5 g/g (HD), respectively, used in each injection mode. Therefore, the study was divided into 4 groups: 3×LD, 3×HD, 5×LD, and 5×HD. SOD enzyme, catalase, and the amount of GSH that has been oxidized/reduced were determined as endogenous antioxidants. Oxidation of cellular macromolecules was assessed by the formation of lipid hydroperoxide (LOOH) and 3-nitrotyrosine (NT).
Short-term memory deficits in animal models were assessed using the novel object recognition test (NOR). A rat was placed in a test area of 80 × 80 cm with 2 identical objects and explored for 5 minutes. After 20 minutes, the rats returned to the testing area in the cage, where one of the objects was replaced with a new one. Calculate the time spent exploring new objects each time divided by the total time. Exploration of significantly more novel objects than familiar objects (score >0.5) was defined as no memory deficit. Statistics used two-way ANOVA analysis and t-test, and a P value <0.05 was considered statistically significant.
Transmission electron microscopy (TEM) shows a CeONP suspension with an average particle size of 10 nm, a homogeneous, agglomeration-free mixture. CeONPs were suspended in a citrate solution and reached the brain at concentrations shown in Figure 2. The cerium content of CeONPs in the brain increased 3.6-fold with the LD dose and 5.8-fold with the HD dose. CeONPs are not metabolized in the brain and accumulate for up to 6 months after administration. No adverse effects were reported at doses of CeONPs or doses 10 times higher.
Cerium oxide nanoparticles. Representative transmission electron microscopy (TEM) image of nanoparticles after injection of citrate solution (average particle size 10 nm). Note that the particles are not agglomerated and are of uniform size. Scale bar = 10 nm.
Distribution of cerium oxide nanoparticles (CeONPs) in the brain. As a method to measure CeONP content, brain cerium content was measured using inductively coupled plasma mass spectrometry (ICP-MS) and inductively coupled plasma generation spectroscopy (ICP-OES) at concentrations shown in a single injection of CeONP (Cerium Labs, Austin, TX). Even animals not exposed to CeONPs had basal levels of cerium in the brains, which were observed in citrate-treated controls. Note the dose-dependent increase in cerium in the brain with concentration of CeONPs. *Significant difference compared with normal saline group, P<0.01.
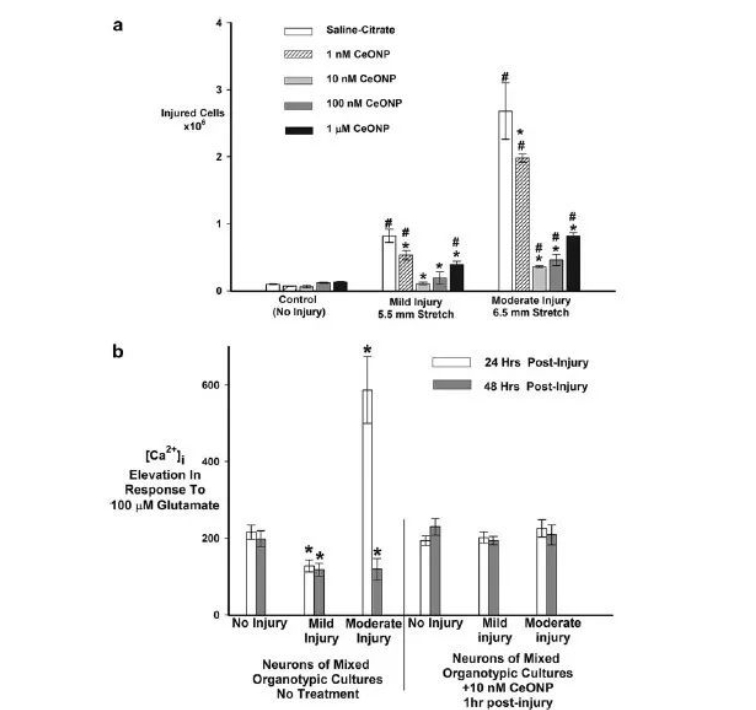
Propanodium iodide uptake (PrI) experiments showed that undamaged cultured cells showed trace amounts of PrI uptake. Mild or moderate injury increased PrI uptake 24 hours later. When cells were treated with CeONPs 1 hour after mild injury, the absorption of PrI was reduced, with 10 nM of CeONP being the most effective dose. At moderate injury levels, 10 nM CeONP reduced PrI uptake by 78%. 1 μM CeONPs for 24 hours reduced PrI uptake less effectively than lower doses.
The [Ca2+]i experiment showed that the average change in [Ca2+] I produced by undamaged neurons after stimulation with 100 μM glutamate was 203±12nM (Figure 3B). The response to glutamate was reduced 24h or 48h after mild injury. Neuronal injury responses to glutamate were significantly increased 24 hours after moderate injury, suggesting increased excitotoxic damage and responses to glutamate were below normal levels 48 hours after injury. Cells treated with CeONPs showed no distortion in glutamate signals 24 and 48 h after mild or moderate in vitro injury. These results indicate that CeONPs can effectively inhibit abnormal glutamate signaling caused by injury and preserve neurological function after trauma.
Cerium oxide nanoparticles (CeONPs) increase neuronal cell survival and improve glutamate signaling after trauma in vitro. In panel a, mixed organotypic brain cell cultures were subjected to in vitro traumatic brain injury (TBI) at mild (5.5 mm stretch) and moderate (6.5 mm stretch) levels. CeONPs were administered at designated concentrations within 1 hour of injury. The control group received saline citrate. Neuronal cell death or injury was quantified by propidium iodide (PrI) uptake 24 hours after injury. Results are expressed as damaged cells/mg protein and are representative of three independent experiments performed in triplicate. #Significant difference compared with undamaged culture, P<0.01. *Significant difference compared with a single injury, P<0.01. For group b, in vitro TBI culture was performed, followed by treatment with 10 nM CeONP for 1 h (right-hand straight bar group) or salt-citrate (left-hand straight bar group). Changes in [Ca2+]i under glutamate stimulation was measured and imaged with a Fura-2 microspectrophotometer. The results are expressed as μ±SE changes in [Ca2+] I under glutamate stimulation. Three independent experiments, with at least 50 neurons per experimental field of view, were performed twice. CeONP treatment blocks the interference of glutamate-stimulated calcium signaling after injury. *Significant difference compared with no damage, P<0.01.
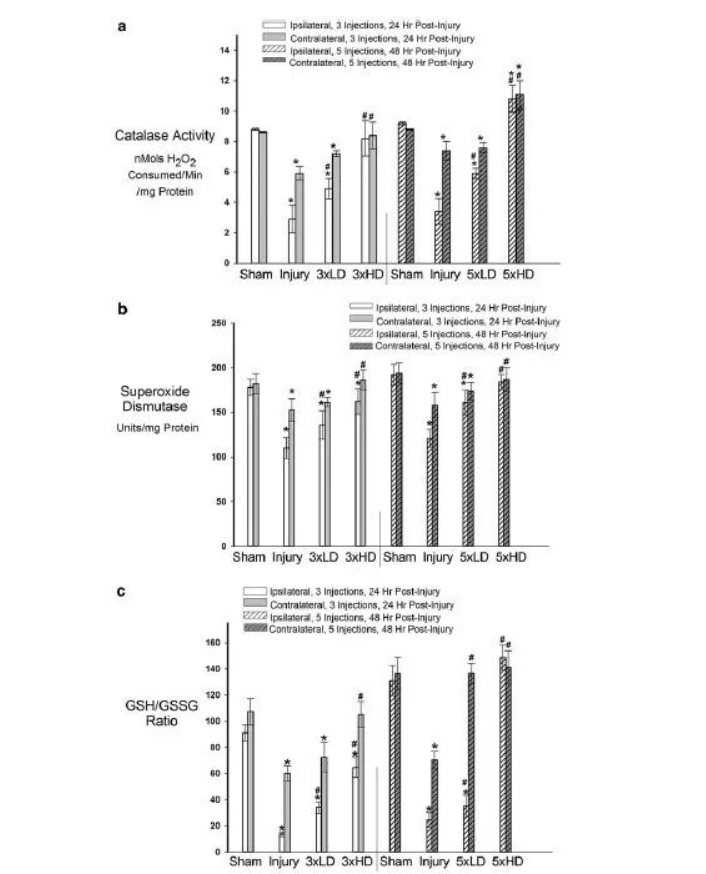
In the in vivo TBI model study, as shown in Figure 4A, the catalase activity on the ipsilateral and contralateral sides of the injured animals was significantly reduced compared with the sham-operated group, consistent with TBI-induced oxidative stress damage. Catalase activity in the CeONP 3×LD group was significantly increased compared with the injury-only group, while the 3×HD drug returned to the level of the sham operation group. There were similar results in the 5× dose group (Figure 4A). This increase may be due to the accumulation of CeONPs in the brain. As shown in Figure 4B, the total SOD activity (copper/zinc, manganese, iron) in both the ipsilateral and contralateral hemispheres was significantly reduced compared with the sham operation group. Most notably the ipsilateral hemisphere. These decreases were also restored after the CeONP injection.
Whether CeONPs can alleviate the damage caused by excessive free radical production by macromolecular substances, the results are shown in Figure 5A. Compared with sham, mLFPI increased LOOH levels 24 and 48 h after injury, indicating oxidative damage to cellular lipids. Three drug injections of CeONPs slowed the increase in LOOH. HD can restore LOOH levels to the sham group in both hemispheres. Five injections had similar results. NT is a product of free radical damage to proteins. NT was significantly elevated in the ipsilateral hemisphere after mLFPI compared with sham. 24 and 48 h increased by 48% and 41% respectively. (Figure 5B) Interestingly, no significant increase in NT occurred contralateral to cortical injury. LD injection of CeONP (3×, 5×) did not significantly affect MT levels. 3×HD reduces NT by 9%, and 5×HD can reduce it to sham levels.
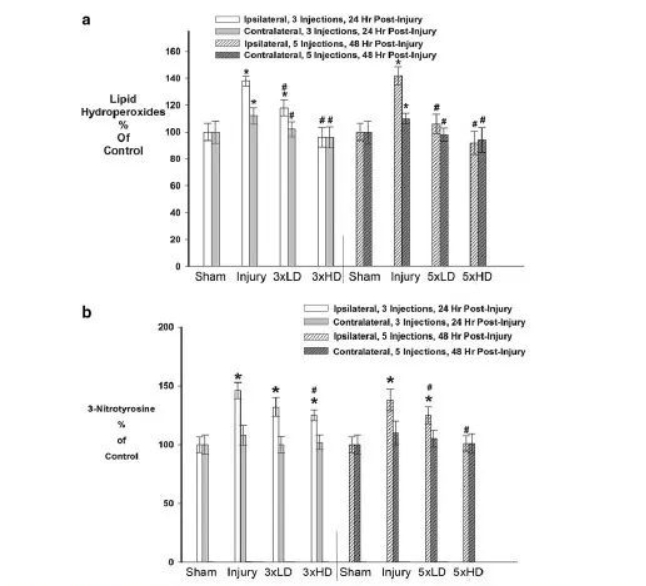
Cerium oxide nanoparticles (CeONPs) reduce oxidative macromolecular damage in mild traumatic brain injury (mTBI) in vivo. Panel a shows that the lipid hydroperoxide content in CeONP-treated rats decreased after mTBI, and the lipid hydroperoxide content in the chronic high-dose group was comparable to that in the sham-operated group. Panel b shows decreased 3-nitrotyrosine levels in CeONP-treated animals. Likewise, chronic high doses reduced levels to those of the sham group. *Significant in sham, p < 0.01; #Significant in injury group, p < 0.01.
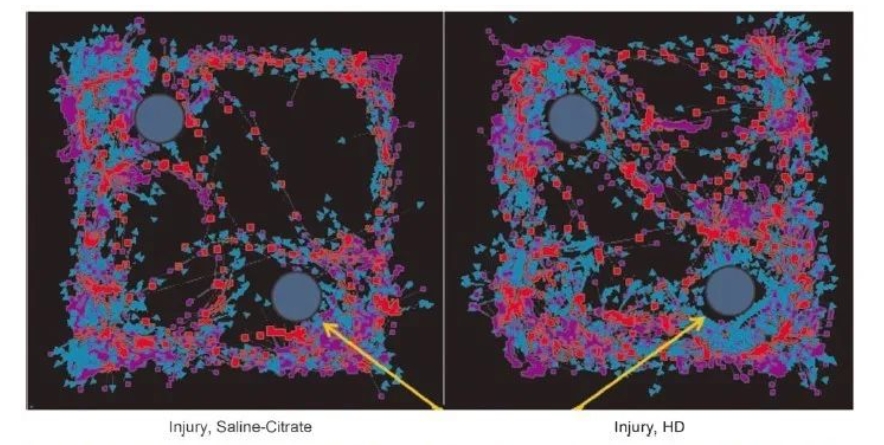
Results for NOR testing control mice (left panel) are shown, with a similar exploration of individual objects suggestive of memory deficits compared to injured mice treated with 5×HD (right panel). In contrast, CeONPs-treated animals showed significantly increased exploration of novel objects, indicating intact memory. Figure 7 shows the results of the calculation of preference for novel objects in different treatment groups. When animals received 5×LD, there was a trend to improve the NOR index by 0.5 (p = 0.0794) compared with the control group. The performance of the animal treatment group in 5×HD The preference for new things was significantly higher than 0.5 (p = 0.0041), demonstrating a similar behavior to the sham operation group, proving that memory was improved.
Mild traumatic brain injury (mTBI) alters object exploration in novel object recognition tests. This figure shows a representative trace of the new object recognition test quantified in Figure 7. Yellow arrows indicate new objects. The blue arrow in the tracking indicates nose point tracking, while the red square indicates body center tracking and the purple square indicates tail tracking. Injured animals treated in the control group (left) spent a similar amount of time on both objects and were therefore unable to differentiate between novel and familiar objects. Animals that received chronic high doses of cerium oxide nanoparticles (CeONPs) showed preferential investigation of novel objects, suggesting improved memory.
Chronic cerium oxide nanoparticles (CeONP) treatment of mild traumatic brain injury (mTBI) improves the performance of novel object recognition (NOR) tests. This chart depicts each treatment group's preference for novel objects. The dashed line represents the preference score for novel objects (indicative of a memory deficit) in animals that are unable to distinguish between novel and familiar objects. The memory of the sham operation group and the chronic high-dose group was intact, and the new object recognition value was significantly higher than 0.5 (p = 0.0383 and p = 0.0041). Injurious memory deficits resulted in a preference for novel objects that were not significantly different from 0.5. There was a progressive difference in memory improvement between the chronic low-dose treatment groups (score >0.5), p = 0.0794. Long-term high-dose animals showed significant improvements in NOR scores that were similar to those in the control group. Data are expressed in μ±SE. *Significant difference compared with 0.5, p < 0.5.
This article demonstrates for the first time that the role of CeONPs in the brain can improve the pathological outcomes of mTBI. Although more biochemical and behavioral studies are needed, this study suggests a new promising treatment for mTBI: a CeONP nanomedicine.
